Once synthesized or expressed, peptides undergo multiple steps of buffer preparation, purification and formulation before reaching their final drug form. Reverse-phase HPLC, ultrafiltration, and lyophilization are common techniques used to achieve the purity and stability required for therapeutic use.
Accurate preparation of buffers, solvents, and excipients is critical at this stage. Inconsistent concentrations or pH deviations can reduce recovery or compromise product stability. Whether during ultrafiltration prep, final formulation blending, or freeze-drying optimization, precision and reproducibility are non-negotiable.
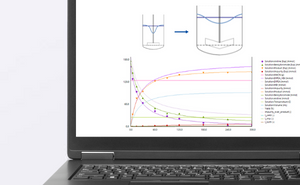
Normal flow filtration and tangential flow filtration play vital roles in downstream bioprocessing, with normal flow filtration mainly used for preparing large volumes of buffer, and tangential flow filtration primarily employed for clarification steps. The in-line monitoring of pH, conductivity, temperature, and UV absorbance enables real-time control of the bioprocess, ensuring optimal conditions that uphold product quality and consistency. This continuous measurement allows for the rapid identification of any deviations, facilitating timely adjustments that enhance process efficiency, increase yield, and maintain product purity throughout downstream processing.
Weighing equipment, pH meters, and UV/Vis spectrophotometers integrated into lab or production environments enable reliable downstream execution and QC in peptide manufacturing. Importantly, when the generated data is centrally collected and managed, it strengthens traceability and supports regulatory documentation.
Additionally, high-precision moisture analysis and water content determination by KF titration support final formulation and stability testing, key in freeze-dried peptide products. Whether during development or commercial QC, laboratory software solutions ensure consistent analytical data for required regulatory compliance and product quality.