Соответствие нормативным требованиям и целостность данных
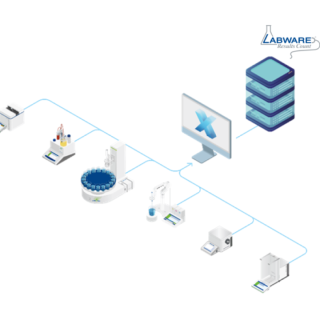
LabX™ обеспечивает соответствие нормативным требованиям и целостность данных, сочетая надежные журналы аудита с улучшенным управлением данными для обеспечения полной прослеживаемости в соответствии с принципами ALCOA++.
Готовность к аудиту в любое время!
Мы предлагаем быстрый и простой доступ ко всем данным, включая централизованное управление пользователями, электронные подписи и подробные журналы аудита в соответствии с такими нормативными требованиями, как 21 CFR, часть 11.