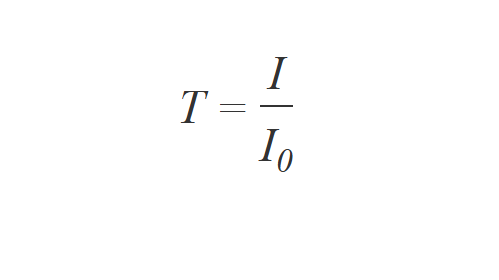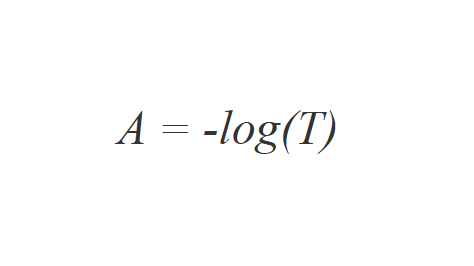Basic Principle of UV/Vis Spectroscopy
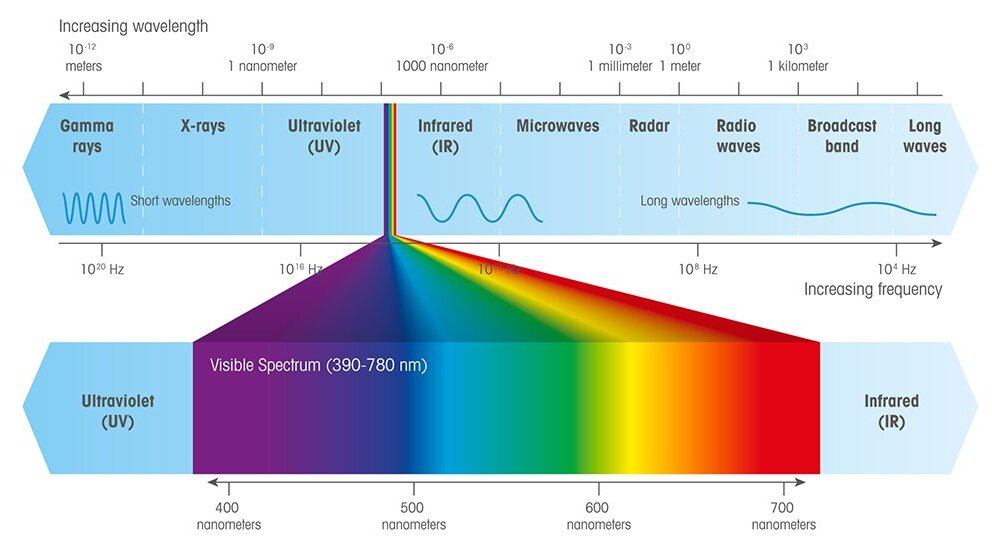
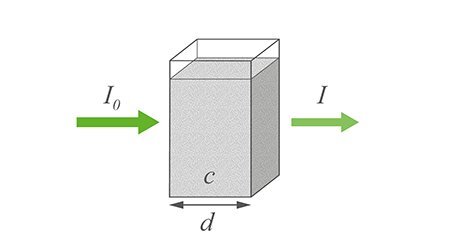
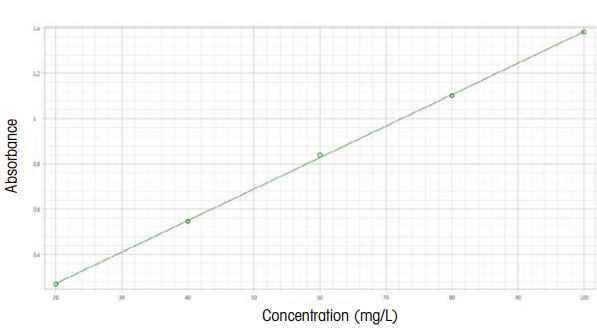
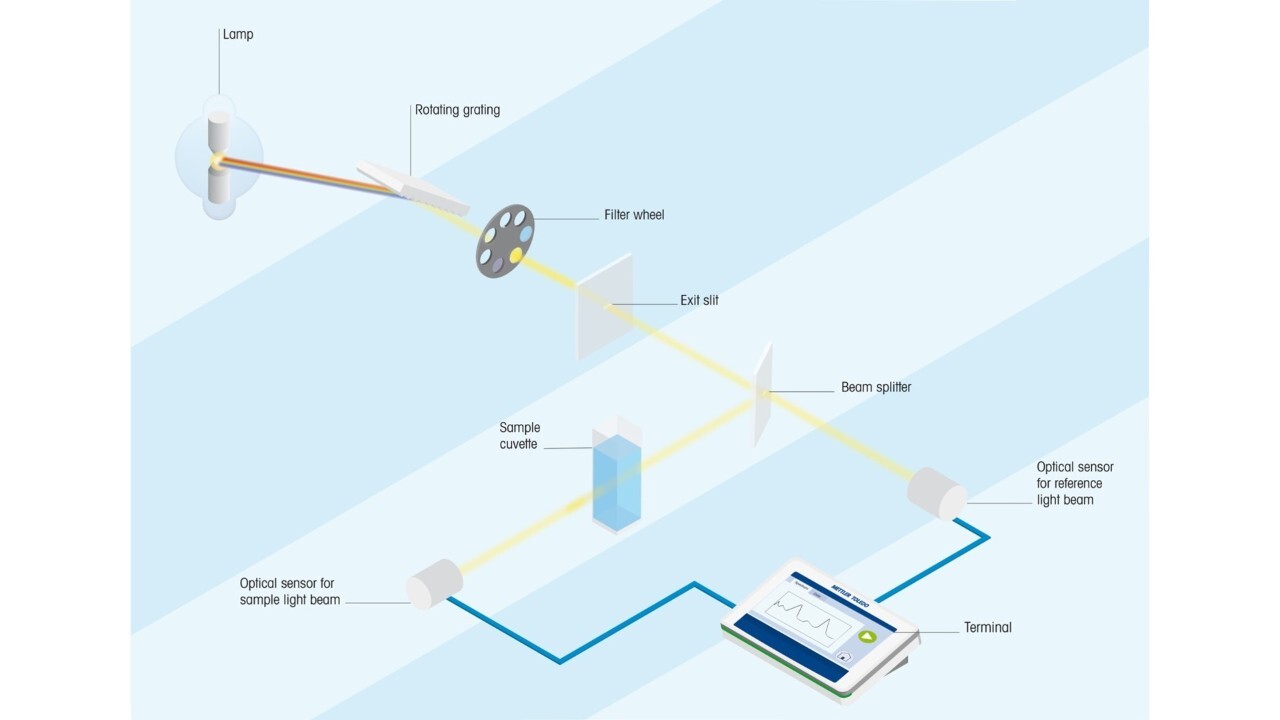
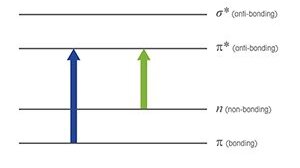
UV/Vis spectroscopy relies on the absorption of specific UV and visible light wavelengths by molecules in a sample. When UV/Vis light interacts with a molecule, a photon can be absorbed if its energy matches the energy difference between the molecule's electronic levels, causing an electron to move to a higher energy state. Wavelengths not absorbed pass through the sample and are detected.
Learn more about the basic principle of UV/Vis spectroscopy, explore applications, and get expert tips for accurate UV/Vis results with our Fundamentals and Applications guide.