Wu, J., Wang, L., Xu, S., Cao, Y., Han, Z., & Li, H. (2023). Sequential hydrogenation of nitroaromatics to alicyclic amines via highly-dispersed Ru–Pd nanoparticles anchored on air-exfoliated C3N4 nanosheets. RSC Advances, 13(3), 2024–2035. https://doi.org/10.1039/d2ra07612h
Hydrogenation of nitroaromatics via green catalytic methods is highly desirable to produce vital alicyclic amines; however, at scale, this is complicated by the different adsorption behaviors of the nitro group and benzene ring. Ru-based catalysts are very effective for the one-step hydrogenation of nitroaromatics to alicyclic amines. Still, the issue of competitive absorption complicates the synthesis and requires harsh reaction conditions. Pd-based catalysts have been shown to have excellent activity and selectivity for the hydrogenation of nitro groups, even under mild conditions. The authors comment that Ru-doped C₃N₄ had previously demonstrated effective aromatic ring hydrogenation. Therefore, for the conversion of nitrobenzene (NB) to cyclohexylamine (CHA), they prepared an air-exfoliated C₃N₄ support containing highly dispersed Ru–Pd dual active sites for the catalytic hydrogenation of the nitroaromatic nitro group and benzene ring, respectively.
A series of physical and spectroscopic investigations were undertaken to fully characterize and define this novel catalyst system's structure–performance relationship. These investigations included determining the C₃N₄ support morphology, the distribution and interaction of the Ru and Pd particles on the catalyst surface, and the dissociation and activation of H₂ under mild conditions. In catalyst performance tests, an NB to CHA reaction was performed to investigate the effect of reaction variables further. A catalyst with 1.5%Ru–1.5%Pd/C₃N₄ reacted at 80 °C and 3 MPa H₂ for 3 h yielded 100.0% NB conversion and 96.8% CHA selectivity.
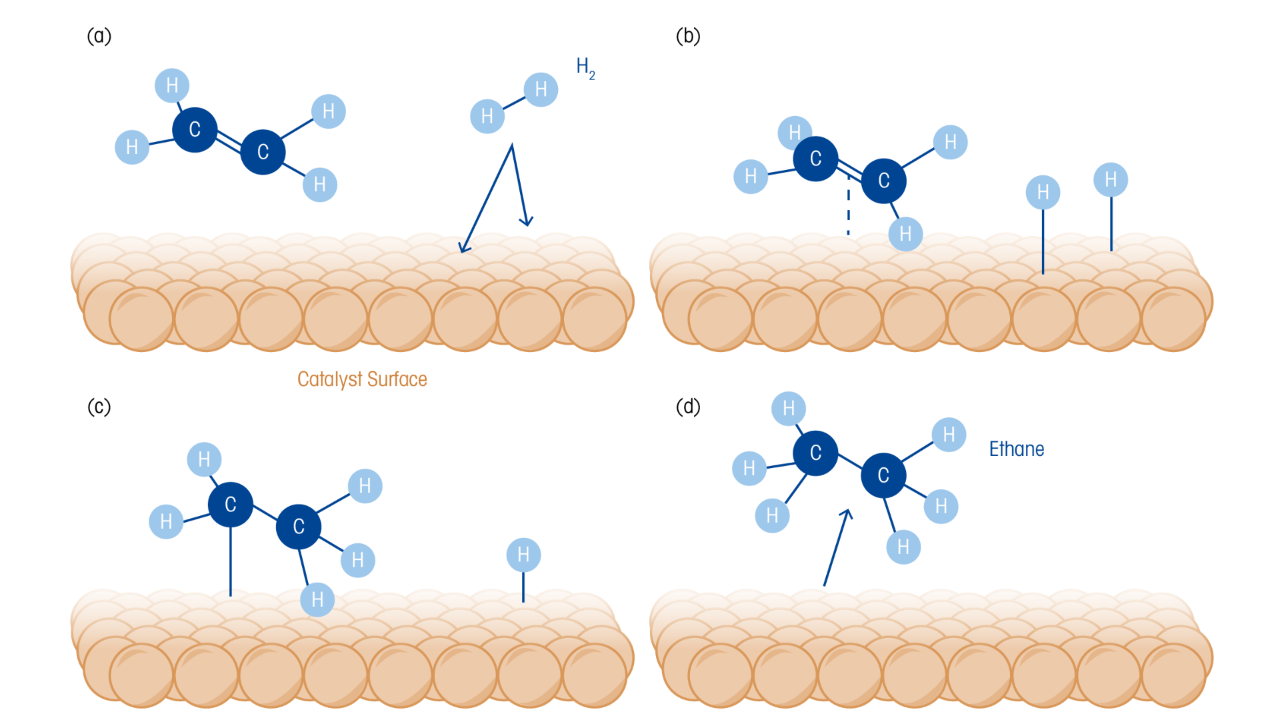
Operando FTIR (ReactIR) was utilized via a custom-built autoclave equipped with a diamond ATR probe for in-situ, real-time measurements to investigate the NB to CHA hydrogenation pathway. The ReactIR measurements exhibited a spectrum with strong peaks at 1350 cm⁻¹ and 1531 cm⁻¹ arising from the symmetric and asymmetric C–NO₂ stretch in NB. As the reaction proceeded, the peak intensity decreased rapidly, indicating a quick conversion of NB. Concurrently, bands at 1606 cm⁻¹ and 1630 cm⁻¹ increased significantly, which was associated with the nitro group transformation to the amine, indicating that aniline was being formed by the direct hydrogenation of NB. Continuing the hydrogenation caused the amine peaks to decrease gradually, reflecting the hydrogenation of the benzene ring to form naphthene. The double peaks at 1606 cm⁻¹ and 1630 cm⁻¹ also contribute to a benzene ring skeleton vibration, weakened with the ring hydrogenation, as the conversion of the AN to CHA took place. By tracking the changes in the key spectral bands as a function of time, ReactIR measurements demonstrated the two-step nature of the hydrogenation process, with the hydrogenation of the nitro group occurring before the rate-determining step, benzene ring hydrogenation.
A reaction pathway was proposed based on combining the operando IR results and GC-MS experiments. Initially, the nitro group is converted into an amino, generating aniline. With additional hydrogenation of the benzene ring, aniline converts to cyclohexylamine. Cyclohexanol and dicyclohexylamine side-products were also observed, resulting from the deamination of AN and condensation of CHA as hydrogenation proceeds. Kinetic studies showed that Pd dominated the hydrogenation of the nitro group, while Ru was dominant for the benzene ring. The authors noted that the activity of the catalyst was greatly improved by the action of the non-dominant metals, which enhanced the activation and dissociation of H₂. Also contributing to the catalyst performance were the highly dispersed Ru–Nₓ and Pd–Nₓ sites at nanoscale separation and the aforementioned metal-assisted hydrogenation.