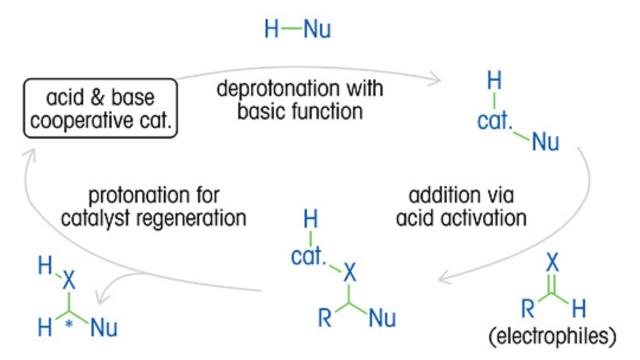
A thorough understanding of reaction kinetics, mechanism, catalytic cycles, and the impact of variables is essential to optimizing enantiomeric ratios and product yield, as well as reducing costs and waste.
To obtain accurate data, precise control of fundamental reaction parameters such as temperature, pressure, reactant dosage rate, and stirring rate is critical. Automated laboratory reactors are widely used in academic and industrial settings to achieve this control. The recent expansion of EasyMax™ lab reactor capabilities to include precise temperature control as low as -78 °C expands the design space for catalytic processes.
Superior reactor control enhances the accuracy of reaction measurements, including the analysis of reagents, reactants, catalyst species, transient intermediates, and by-product impurities. Real-time tracking of reaction species enables the collection of data-rich experiments that can inform the development of kinetic parameters and support proposed reaction mechanisms, including catalytic cycles.
In-situ, real-time FTIR and Raman spectroscopy are established techniques for gaining a deeper understanding of catalytic reactions. ReactIR™ and ReactRaman™ spectrometers were designed for chemical reaction analysis, offering a range of insertion probes and flow cells that can handle the wide range of temperatures and pressures often required for catalytic reaction investigations.
Automated sampling of chemical reactions enables increased productivity by addressing challenges on two scales:
- Manual sampling tools, such as pipettes and syringes
- The manual sampling process itself, with challenging temperature, pressure, or air-sensitive reactions
EasySampler™ is an automated reactor sampling system designed for inline acquisition, quenching, and dilution of reaction samples. It allows samples to be retrieved under reaction conditions and eliminates manual sampling process challenges.
DirectInject-LC™ delivers in-situ, real-time analysis of chemical reactions using chromatographic measurements. The automated system addresses the inherent difficult issues associated with chromatography, such as reaction sampling, quenching, dilution, and interfacing with the chromatograph. DirectInject-LC differentiates and measures key species as the reaction proceeds, yielding an in-depth understanding of reaction kinetics, mechanism, and the effect of variables on reaction outcome. DirectInject-LC interfaces with HPLC-MS, UHPLC, and chiral HPLC systems for advanced separation and analysis capability.
When scaling up catalytic reactions, recent advances in chemical reaction modeling provide the information necessary for safer, higher-yield reactions with fewer impurities. Dynamic modeling of experimental data also offers deeper insights into reaction kinetics and the effect of reaction variables.