GLP-1 Peptide Solutions for Discovery, Development, and Quality Control
Lab Products for Accelerating GLP-1 Peptide Progress
GLP-1 peptide therapeutics demand precision across synthesis, purification, characterization, and quality control. METTLER TOLEDO offers a portfolio of bench-top instruments, software, and services designed to deliver traceable, reproducible measurements that support GLP-1 discovery through scale-up and release testing. Our solutions enable integrated workflows, efficient method transfer, and data integrity across multi-site programs.
We address key challenges in GLP-1 peptide development by ensuring accurate weighing and dosing to preserve peptide yield and reduce batch failures. Our approach includes reliable analytical tests for purity, identity, and degradation products, allowing you to conduct reproducible stability and formulation testing under controlled conditions. We offer laboratory software that enables scalable workflows, protects data integrity, and supports the acceleration of method transfer.
Related Solutions
Therapeutic Peptide Development: From R&D to Scale-Up
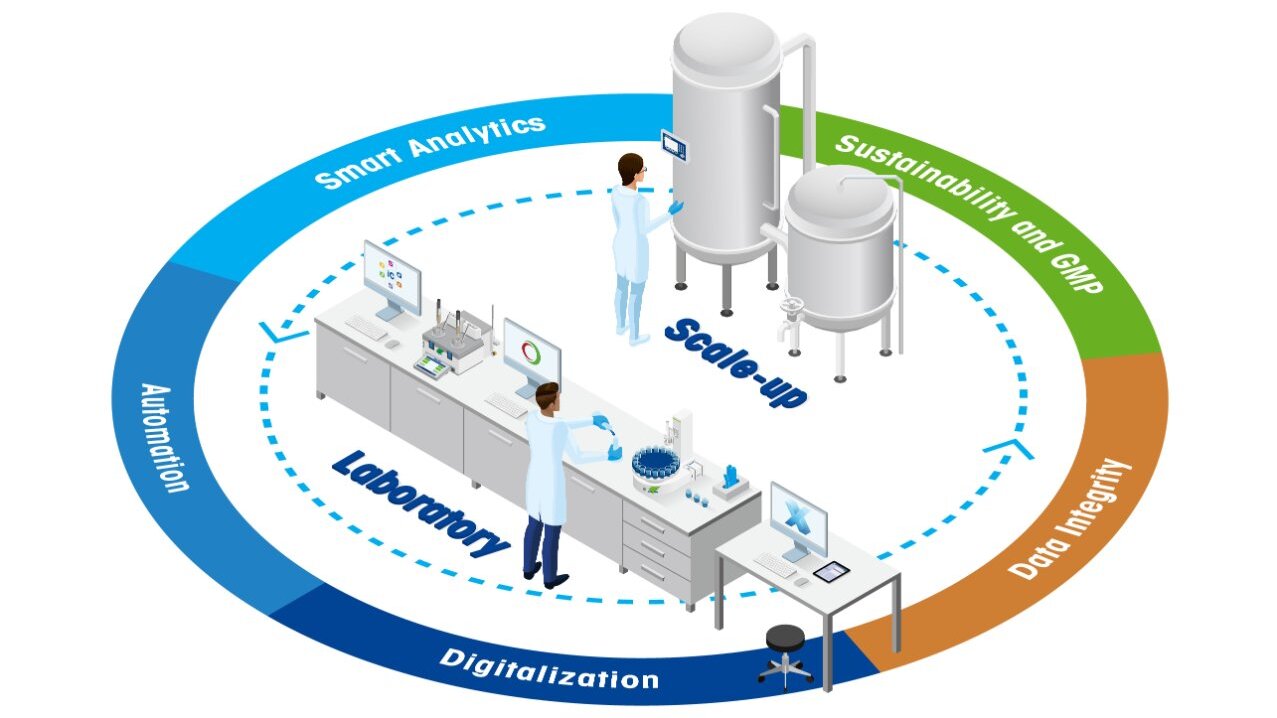
Operational areas:
1. Research
2. Early Stage Development
3. Late Stage Development
4. Piloting and Scale-up
5. Regulatory and Compliance
Our instruments support various stages of GLP-1 workflows, including precision balances for accurate weighing, automated liquid handlers to reduce errors, and spectrophotometers and titrators for potency and concentration analysis. Thermal analysis and stability instruments support formulation and aging studies, while our chromatography-compatible tools aid purity testing.
Methods for GLP-1 Peptide Synthesis and Bioprocessing
Related Know-How
FAQs
Which instruments are most relevant for GLP-1 peptide analysis?
The most commonly used instruments for GLP-1 peptide analysis include precision balances and automated liquid handlers, spectrophotometers, titration systems, and thermal analysis instruments. These instruments collectively support critical workflow stages, including synthesis, purification, analytical characterization, and stability testing, ensuring the precise and reliable development of GLP-1 peptides.
Can METTLER TOLEDO systems integrate end-of-the-end workflows?
Our analytical instruments are designed to interconnect through compatible software platforms, enabling multiparameter workflows and facilitating traceable data exchange.
What training and validation support are available?
We provide operator training, preventive maintenance, and IQ/OQ validation packages to support reliable instrument performance.
Can METTLER TOLEDO solutions scale with growing production demands?
Our modular instrument designs and configurable software allow laboratories to expand capacity without overhauling existing systems. Whether moving from milligram-scale research to gram-scale pilot production or full-scale manufacturing, our solutions adapt through add-on modules, parallel processing, and networked control. This scalability helps companies accelerate time-to-market while protecting their initial capital investments.
Are METTLER TOLEDO's instruments compliant with industry regulations?
Yes, our laboratory systems meet key regulatory requirements, including FDA 21 CFR Part 11, GMP guidelines, and ISO standards for analytical instrumentation. We offer validation packages and services to support installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). Thorough documentation and audit trails ensure traceability and seamless regulatory inspections.
How does integration and software support GLP-1 workflows?
Ensure regulatory compliance, protect data integrity, and improve efficiency in Pharma and BioPharma labs with LabX™ software. Our connected software platforms help you centralize your measurement data, automate calculations, and maintain auditable records that support regulatory review and simplify method transfer.










