What Is the Scope of the Pharmacopoeia General Chapters
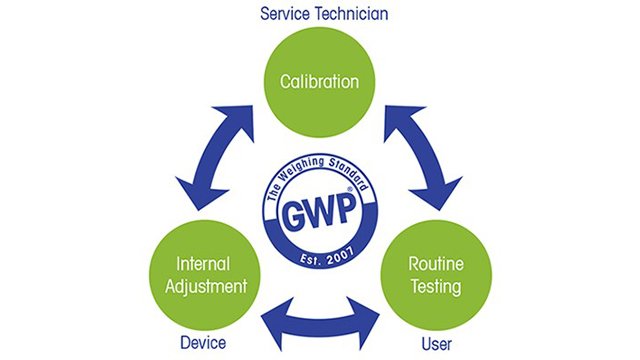
The Pharmacopoeia General Chapters address equipment performance and introduce measures that must be carried out periodically to optimize balance performance:
- Calibration must be documented in a calibration certificate and must include measurement uncertainty.
- Repeatability and Accuracy are the two performance checks that must be executed between calibration intervals.
- Highlight the importance of “as found” and “as left”.
Furthermore, the Pharmacopoeias recommend applying adjustment by built-in weights.
Please note: These chapters and the requirements in them only apply to pharmaceutical quality control. They are not applicable to pharmaceutical manufacturing.