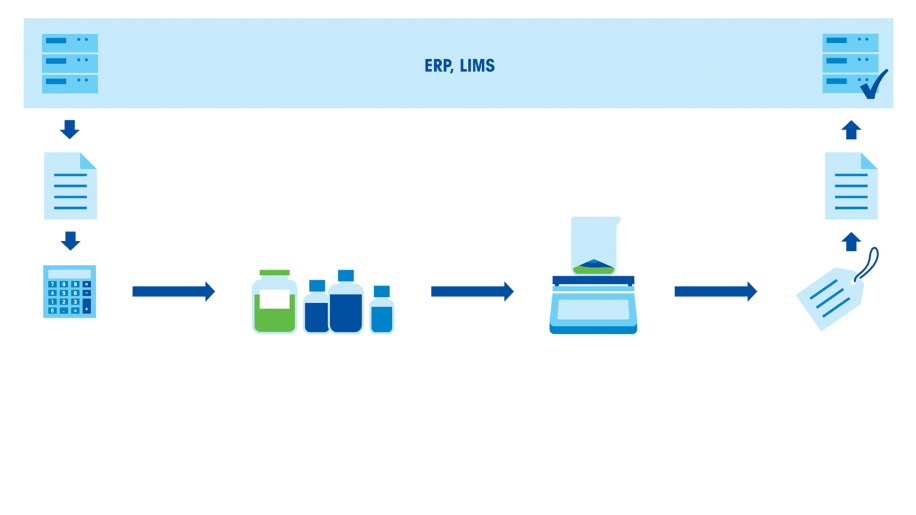
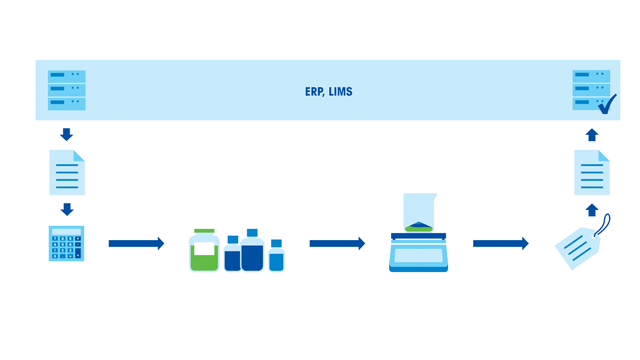
Typical sources of error in any weighing process include: Lack of weighing tolerances, use of inappropriate instrumentation, sample spills, temperature variations, air drafts, documentation errors and insufficient scientist skills. As formulation processes involve multiple weighing steps, the combined errors can become significant.
Product Consistency
Every formulation is developed to meet certain criteria, whether it be taste, color, or drug dosage. Without consistency in the formulation, the product will fail to meet expectations, leading to dissatisfied customers, and may even pose a risk to health and/or safety. It is therefore essential to ensure that the weight of each formulation ingredient meets the process tolerances defined in the formulation SOP. The balance used must be able to fulfil the desired process accuracy requirements and should have appropriate quality assurance measures in place to ensure this.
Recipe Complexity
Cosmetics formulations often consist of 50-100 different components, requiring a great deal of effort and concentration from the analyst. Overdosing just one component requires recalculations and adjustments to be made to the ingredients, or the entire formulation must be started again from scratch. The risk of an error occurring can be significantly reduced if the formulation workflow includes steps for the scientist to confirm:
- The next material to be added is the right one
- The amount required of the next material
- The tolerances for the amount to be added
Accurate Weighing of Critical Components
For small quantities of critical or expensive ingredients, it may be necessary to use a second balance with a higher readability and greater accuracy, particularly where there is a large difference between the individual ingredient amounts and the total target mass. Having two balances occupies more lab space, creates extra steps in the workflow, increases the process uncertainties and necessitates a higher investment.
Documentation
To ensure traceability, formulation documentation typically includes formulation ID, substance ID, target mass, tolerances, and actual amount dosed, as well as basic information such as date, time, and user ID. Any recalculations and adjustments must also be documented. Manual recording of all this information carries a huge risk of human error. If there is any mistake in the formulation, it may be impossible to find the source of the error if the data has been recorded incorrectly.