Yetra, S. R., Schmitt, N., & Tambar, U. K. (2022). Catalytic photochemical enantioselective α-alkylation with pyridinium salts. Chemical Science, 14(3), 586–592. https://doi.org/10.1039/d2sc05654b
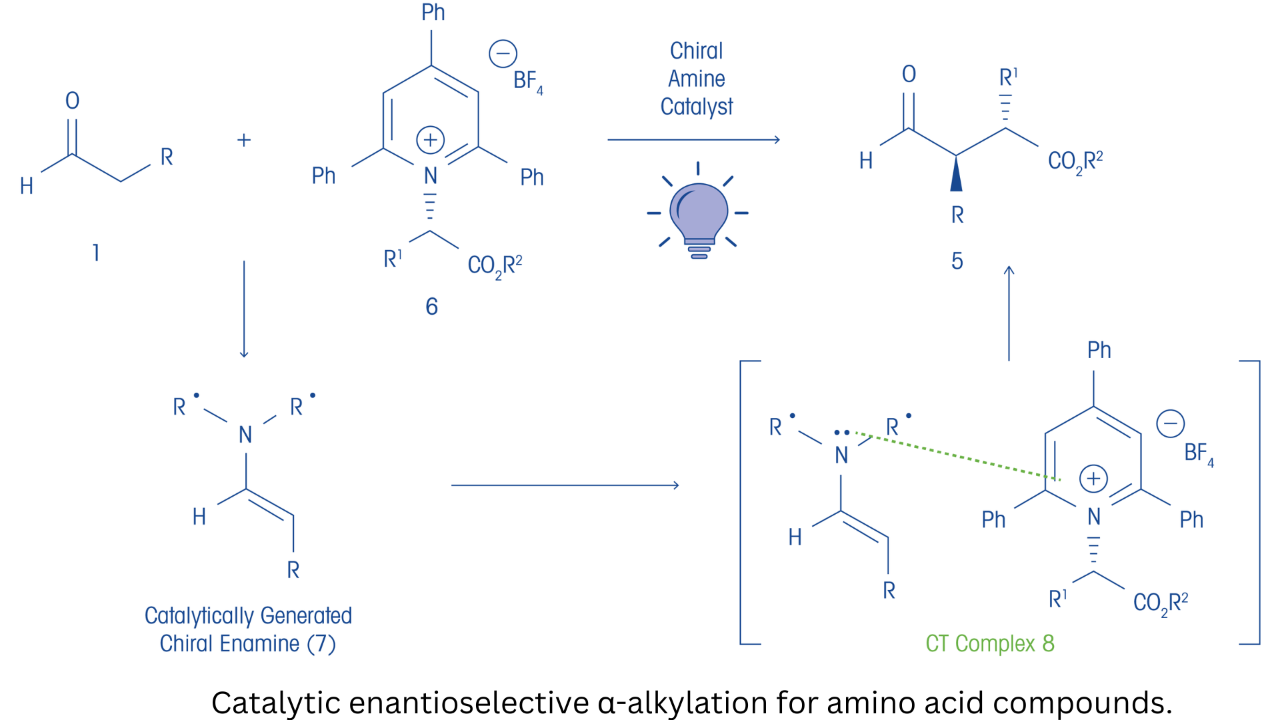
The authors commented that alkyl halides and sulfonates are frequently employed alkylating agents used in asymmetric catalysis for the enantioselective α-alkylation of enolates. Their interest was in developing a photochemical process for enantioselective alkylations that uses renewable and sustainable sources of alkylating reagents such as amino acid-derived substrates. Given the low electron acceptance capability of amino acid derivatives in enolate alkylations, the challenge was to develop a means to activate these compounds. Based on earlier work in the literature, the authors postulated that using amino acid-derived pyridinium salts as alkylating agents would be effective, given pyridinium salts are known to be used as radical precursors in enantioselective α-alkylations. They proposed that pyridinium salts form ground-state complexes with catalytically generated, electron-rich chiral enolate equivalents. In an extensive series of experiments, they showed that an electron-deficient Katritzky salt derived from the 2,2,2-trifluoroethyl ester of glycine reacted under conditions using a chiral amine catalyst, 2,6-lutidine, and 427 nm irradiation, provided the desired α-alkylation product.
Additional work showed that using a Lewis basic medium, such as dimethyl acetamide, improved yield (to 40%) and provided excellent enantiomeric excess (ee. 92%). Furthermore, using additives such as sodium iodide that improve ground-state complexation of the reaction components resulted in yields of 75% with 92% ee. Through in-depth mechanistic studies, they postulated that the catalytic enantioselective reaction may proceed simultaneously via an in-cage radical combination mechanism and a radical chain mechanism. The researchers went on to understand the photocatalytic reaction scope, including using the process in the total synthesis of the lignan natural products (−)-enterolactone and (−)-enterodiol.
A key observation in their work was the critical importance of controlling reaction temperature. Performing these reactions at room temperature negatively affected the enantioselectivity, and maintaining 92% ee required running the reaction at a temperature of 4 °C. Temperature control was challenging since the reaction was continually irradiated with a light source near the vessel. For this reason, the researchers used an EasyMax 102 system. In an article highlighting Professor Tambur’s work on catalytic photochemical enantioselective α-alkylation using pyridinium salts (Synform, 2023/06, A100-A105), he comments: “We finally purchased the EasyMax 102 Advanced Thermostat system from Mettler-Toledo AutoChem, Inc. This turned out to be the most important purchase for the success of the project. Although the EasyMax had never been used for photochemical reactions, we identified two key features of this instrument. First, it enables the maintenance of a constant low reaction temperature for long times. Second, the instrument has a clear window into the reaction chamber, which is typically used to view
into the reaction, but we identified this as an opportunity to shine light from a lamp at a controlled distance without impacting the reaction temperature. To our delight, the EasyMax provided a new level of consistency in our results.”