Wang, Y., Yu, J., Wang, Y., Chen, Z., Dong, L., Cai, R., Hong, M., Long, X., & Yang, S. (2020). In situ templating synthesis of mesoporous Ni–Fe electrocatalyst for oxygen evolution reaction. RSC Advances, 10(39), 23321–23330. https://doi.org/10.1039/d0ra03111a
In support of electrocatalyst development, ReactRaman provides detailed information on bonding within and at the surface of the mesoporous Ni–Fe electrocatalyst.
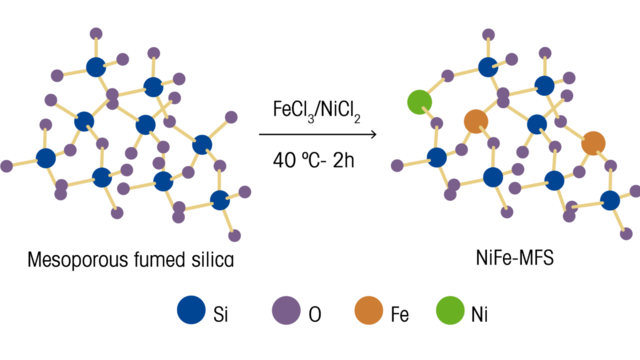
The authors comment on the importance of developing electrocatalysts for use in oxygen evolution reactions (OER). These electrocatalysts must have specific key efficiency characteristics, such as active sites that are evenly distributed and readily available across the surface of the catalyst while being cost-effective and sustainable. To meet these goals, they researched and developed a method to prepare a mesoporous fumed silica (MFS) framework that dispersed Ni²⁺ and Fe³⁺ using a straightforward approach. This method uses commercially available MFS as a 3D support to attach the metal ions. By etching the MFS metal-impregnated structure with KOH, the Ni−Fe−O electrocatalyst formed has key characteristics for OER, such as good charge transfer ability, large electrochemical active surface area, and overall excellent stability.
A series of NiFe-MFS catalysts were synthesized with different mole ratios of metal-ion aqueous solutions. A series of techniques were used to develop a detailed understanding of the microstructure of these electrocatalysts. This included transmission electron microscopy to investigate the nanostructure, energy-dispersive X-ray to map the Ni, Fe, Si, and O element distribution, and X-ray diffraction to analyze the crystallinity of the samples. Morphology was studied by emission scanning electron microscopy; X-ray photoelectron spectroscopy analyzed the element binding energy, and elemental analysis was performed using an inductively coupled plasma-atomic emission spectrometer. Surface area and structure porosity were investigated using nitrogen gas adsorption-desorption measurements.
The bonding of the Ni/Fe metals to the fumed silica support was analyzed and verified with ReactRaman spectroscopy. For undoped MFS, bands at 345–450, 575, 750, 973, and 1070 cm⁻¹ are observed, arising from a series of Si−O−Si and Si−OH vibrational bonds. For samples that are impregnated with high iron content, bands at 332, 495, and 1163 cm⁻¹ are observed arising from O–Fe–O bending, Fe–O–Si bending, and the Fe–O–Si asymmetric stretch, respectively. These observations indicated that the iron was effectively incorporated into the silica lattice. In contrast, when nickel was impregnated into the fumed silica, the 973 cm⁻¹ surface Si−OH stretching band was significantly weakened, and no additional bands were observed. Evaluating the X-ray photoelectron and Raman measurements led to the conclusion that, whereas Fe³⁺ prefers to insert into the framework of the fumed silica and forms Fe–O–Si bonding, Ni²⁺ binds covalently with Si−OH groups on the fumed silica surface.
A series of electroanalysis studies were performed that showed the importance of the metal ion ratio to performance and that Ni and Fe bonding at an optimal content led to optimum OER efficiency and improved reaction kinetics. The 1Ni1Fe-MFS sample demonstrated the highest OER intrinsic activity, while 2Ni1Fe-MFS catalyst had the larger double-layer capacitance and electrochemically active surface area. A series of spectroscopic investigations were carried out to determine the changes in the catalyst after OER. They showed that in the presence of the KOH, the Si was etched, exposing Ni and Fe elements, which were the OER active centers. Further work showed that even after long-term electrical operation, 2Ni1Fe-MFS catalyst remains both highly efficient and stable during the OER process, in comparison testing with IrO₂ and RuO₂ electrodes.