Naleving van regelgeving en gegevensintegriteit
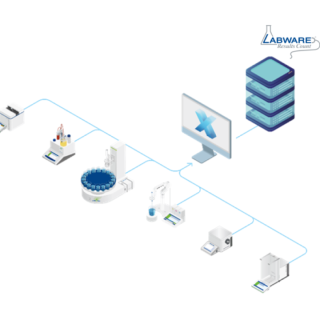
LabX™ ondersteunt compliance met wet- en regelgeving en data-integriteit door robuuste audittrails te combineren met verbeterd databeheer voor volledige traceerbaarheid, in overeenstemming met de ALCOA++-principes.
Audit-klaar, op elk moment!
We bieden snelle, makkelijke toegang tot alle gegevens, met gecentraliseerd gebruikersbeheer, elektronische handtekeningen en uitgebreide audittrails, in overeenstemming met regelgeving zoals 21 CFR Deel 11.